ZEISS MyoCare (CARE) – Clinical Evaluation of MyoCare in Europe (CEME) Study
1. Study Design & Scope
-
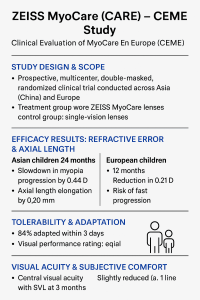
A prospective, multicenter, double-masked, randomized clinical trial conducted across Asia (China) and Europe.
-
European cohort (CEME Study):
-
Enrolled around 234–304 Caucasian children aged 6–13 years.
-
Eligibility: cycloplegic spherical equivalent (SE) between –0.75 D and –5.00 D, with past annual progression of at least –0.50 D.
-
-
Treatment group wore ZEISS MyoCare spectacle lenses with cylindrical annular refractive elements; control group wore single-vision lenses (SVL).
2. Efficacy Results: Refractive Error & Axial Length
Asian Children (2-Year Data)
-
After 24 months:
-
Myopia progression slowed by 0.44 D compared to SVL.
-
Axial length (AL) elongation reduced by 0.20 mm.
-
-
Variant MyoCare S:
-
Slowed progression by 0.41 D and AL by 0.17 mm.
-
Caucasian (European) Children (1-Year Data)
-
After 12 months:
-
Myopia progression slowed by 0.21 D.
-
AL growth reduced by 0.14 mm.
-
Significant reduction in “fast progression” cases (progression ≥ 0.50 D or AL ≥ 0.20 mm).
-
-
ZEISS MyoCare showed consistent benefit regardless of prior progression history.
3. Tolerability & Adaptation
-
Rapid adaptation observed: 84% of lens wearers adapted within 3 days in Europe.
-
Visual performance ratings:
-
95% rated distance vision as “good or very good”.
-
93% rated near vision positively.
-
96% reported good vision during sports and daily activities.
-
4. Visual Acuity & Subjective Comfort
-
At dispensing:
-
Central visual acuity was slightly better with MyoCare than SVL (not clinically significant).
-
Peripheral visual acuity was about one line lower with MyoCare.
-
-
At 3 months:
-
Central vision and subjective adaptation scores matched SVL.
-
Peripheral vision reduction persisted but remained within acceptable clinical limits.
-
-
Adaptation scores improved over time, showing equivalence to SVL by 3 months for most parameters.
Summary Table: Key Findings
| Population | Duration | SE Slowdown | AL Slowdown | Fast Progression Reduced | Adaptation Rate | Visual Tolerance |
|---|---|---|---|---|---|---|
| Asian children | 24 months | 0.44 D | 0.20 mm | — | — | — |
| European children | 12 months | 0.21 D | 0.14 mm | Yes | 84% within 3 days | 93–96% rated vision “good or very good” |
| Visual comfort | 3 months | — | — | — | Improved, equal to SVL | Slight peripheral VA decrease (1 line) |
Why These Findings Matter
-
Cross-population efficacy: ZEISS MyoCare demonstrated consistent benefits across Asian and European children, proving applicability across diverse ethnic groups.
-
High compliance potential: Rapid adaptation and strong subjective satisfaction indicate high likelihood of long-term daily wear.
-
Clinically acceptable trade-offs: Slight reductions in peripheral vision were observed, but overall visual acuity and comfort matched single-vision standards within a few months.
You can place an order or ask any questions. Please feel free to contact us at sales@precedenceresearch.com |+1 804 441 9344