The vaccine CDMO market is gaining momentum with a compound annual growth rate (CAGR) of 9.10% forecasted between 2025 and 2034. This growth is largely propelled by increased funding from both public and private sectors aimed at accelerating vaccine design innovations and optimizing production processes. With rising demand for vaccines to combat infectious diseases and the urgent need for rapid pandemic response, CDMOs are playing a pivotal role in expediting vaccine availability worldwide.
Vaccine CDMO Market Key Highlights
-
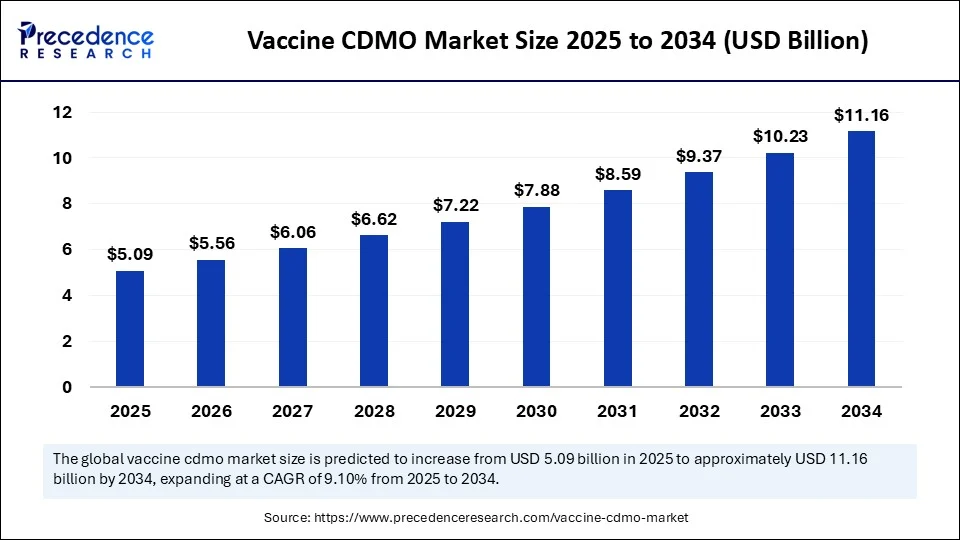
The vaccine CDMO market size was USD 4.67 billion in 2024 and is expected to more than double by 2034 to USD 11.16 billion.
-
North America led the market in 2024, supported by advanced healthcare infrastructure and strong regulatory frameworks.
-
Asia Pacific is the fastest-growing region, benefiting from cost efficiencies and expanding government support.
-
The U.S. market alone was valued at USD 1.47 billion in 2024, projected to reach USD 3.58 billion by 2034.
-
mRNA vaccine production dominates the modality segment due to rapid development successes during the COVID-19 pandemic.
-
Mammalian cell culture is the leading expression platform, while bacterial/E. coli is the fastest-growing segment.
-
Commercial-scale manufacturing commands the largest revenue share, although clinical-scale is rapidly evolving.
-
Liquid vaccine formulations dominate due to patient compliance and stability, with lyophilized vaccines growing fast for improved shelf life.
Get this report to explore global market size, share, CAGR, and trends, featuring detailed segmental analysis and an insightful competitive landscape overview @ https://www.precedenceresearch.com/sample/6679
Vaccine CDMO Market Revenue Breakdown and Forecast
| Parameter | Value |
|---|---|
| Market Size (2024) | USD 4.67 Billion |
| Market Size (2025) | USD 5.09 Billion |
| Market Size (2034) | USD 11.16 Billion |
| CAGR (2025-2034) | 9.10% |
| Leading Region | North America |
| Fastest Growing Region | Asia Pacific |
Artificial intelligence (AI) is revolutionizing vaccine development and manufacturing by enhancing research efficiencies and production quality. Machine learning models assist in predicting optimal antigenic epitopes and vaccine candidate immunogenicity, while generative AI techniques improve immunogen stability. Moreover, AI-driven predictive analytics streamline clinical trials by designing effective protocols and selecting appropriate patient cohorts, thus shortening timelines.
On the manufacturing floor, AI analyzes production data to detect anomalies and optimize processes, which results in higher productivity, less downtime, and consistent vaccine quality output. Integration of AI enables CDMOs to meet increasing demand with speed and precision, reinforcing their crucial role in global vaccine accessibility.
What Market Growth Drivers Are Shaping the Vaccine CDMO Industry?
Several key factors underpin the vaccine CDMO market’s robust expansion. First, heightened public and private investments promote innovation in vaccine production technologies and capabilities. Second, the growing complexity of vaccines, especially next-generation modalities like mRNA and viral vectors, compels biopharmaceutical companies to outsource to specialized CDMOs possessing advanced infrastructure and technical expertise. Third, the need to rapidly respond to infectious disease outbreaks and immunization programs worldwide fuels demand. Lastly, supportive regulatory environments in developed regions encourage partnerships and capacity expansion.
What Opportunities and Trends Are Defining the Vaccine CDMO Market Now?
-
How is the shift to personalized and complex vaccine modalities opening doors for CDMOs?
-
Will expanding immunization programs in emerging markets drive demand for scalable vaccine manufacturing solutions?
-
How are public-private partnerships influencing CDMO investments and technological advancements?
-
Can convergence of cancer vaccines and immuno-oncology therapies shape future CDMO service offerings?
Regional and Segmentation Highlights
North America’s dominance stems from its developed healthcare infrastructure, stringent but clear regulatory standards, and concentration of biotech firms and research institutions. Asia Pacific’s rapid growth is driven by cost advantages, skilled labor, and expanding government-backed vaccine initiatives. Europe, Latin America, and Middle East & Africa are steadily growing with increasing vaccine development activities and infrastructure enhancements.
Segmentation insights
-
Modality: mRNA vaccines lead due to successful COVID-19 applications.
-
Expression Systems: Mammalian cell culture dominates, while bacterial/E. coli grows fastest for simpler proteins.
-
Manufacturing Scale: Commercial scale commands the market share; clinical scale is rising.
-
Dosage Form: Liquid formulations are preferred for stability and compliance; lyophilized forms gain momentum for their storage benefits.
Which Are the Leading Companies Driving Innovation and Capacity?

- Lonza
- Catalent
- Thermo Fisher Scientific
- Siegfried (formerly Swiss fill & finish or Siegfried Switzerland)
- Wacker Biotech
- Alcami
- Virchow Biotech
- BioNTech Manufacturing
- Emergent BioSolutions
- Alcami
- Novasep
- Samsung Biologics
- Boehringer Ingelheim BioXcellence
- Fujifilm Diosynth Biotechnologies
- Recipharm
- Almac Group
- Vetter Pharma
- Thermogenesis
- AGC Biologics
- Richter-Helm BioLogics
Challenges and Cost Pressures Facing the Market
The vaccine CDMO industry grapples with evolving regulatory compliance that raises operational costs due to the need for continuous investments in facility upgrades, specialized equipment, and workforce training. Smaller CDMOs face challenges in allocating resources to meet diverse global standards, which can hinder competitiveness. Additionally, maintaining cost-efficiency while scaling complex vaccine production remains an ongoing pressure.
Case Study Snapshot: mRNA Vaccine CDMO Expansion
The COVID-19 pandemic highlighted the critical role of CDMOs in enabling rapid mRNA vaccine production. Leading CDMOs swiftly upgraded facilities and expertise, facilitating fast manufacturing scale-up and global distribution. The success set a new industry benchmark, driving further investments in mRNA platforms and partnership models for future vaccine development.
Read Also: Copper Hydroxide Market
You can place an order or ask any questions. Please feel free to contact us at sales@precedenceresearch.com |+1 804 441 9344