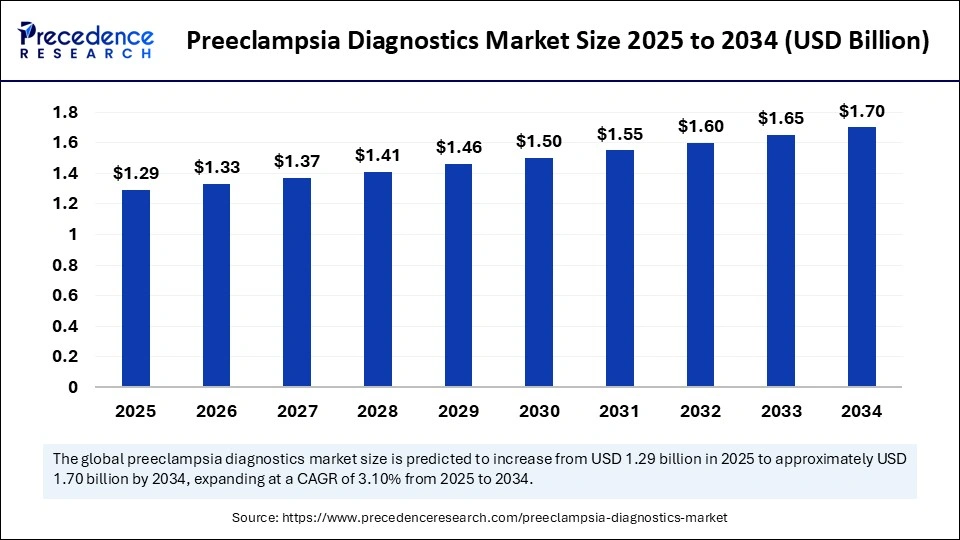
The global preeclampsia diagnostics market is projected to grow from USD 1.29 billion in 2025 to USD 1.70 billion by 2034, expanding at a CAGR of 3.10%. This robust growth is fueled by increasing maternal health awareness, advances in point-of-care diagnostic technologies, and expanding prenatal screening initiatives worldwide.
Preeclampsia Diagnostics Market Key Insights
-
The market size was valued at USD 1.29 billion in 2025, expected to reach USD 1.70 billion by 2034.
-
North America led the market in 2024, holding a 43% share, supported by advanced healthcare infrastructure.
-
Asia Pacific is the fastest-growing region due to rising investments in healthcare services and maternal diagnostics.
-
The biochemical marker assays segment held about 40% market share in 2024, with high accuracy in early detection.
-
Hospitals and maternity clinics accounted for nearly 50% of market share as major end-users of diagnostic services.
-
Leading companies include Thermo Fisher Scientific, F. Hoffmann La Roche Ltd., and PerkinElmer Inc., dominating nearly 50% of the market.
What is propelling the market growth at a steady CAGR?
The market’s growth is primarily driven by the rising prevalence of hypertensive disorders during pregnancy, notably preeclampsia, which affects 2%-8% of pregnancies globally. According to the WHO, preeclampsia causes approximately 46,000 maternal deaths and nearly 500,000 fetal and newborn deaths annually. Increasing government initiatives for early prenatal screening, technological developments in diagnostic assays, and expanding public health awareness are critical growth enablers.
Which technologies and test types dominate the preeclampsia diagnostics landscape?
The biochemical marker assays segment is the largest revenue contributor, favored for its clinical accuracy in identifying preeclampsia risks during early pregnancy stages. Immunoassays, representing 45% of the market share, are widely adopted due to their high sensitivity in detecting biomarkers such as placental growth factor (PlGF) and soluble fms-like tyrosine kinase-1 (sFlt-1). Meanwhile, point-of-care diagnostic kits are gaining rapid traction for their ability to provide affordable, accessible, and timely results, especially in low-resource settings.
How is Artificial Intelligence (AI) transforming preeclampsia diagnostics?
AI plays a transformative role by enabling predictive risk assessment through analysis of complex patient data, including blood biomarkers, maternal history, and placental imaging. AI-driven models, such as those developed by the University of Oxford and King’s College London, have demonstrated high accuracy in early preeclampsia prediction.
Additionally, AI integration within prenatal care supports personalized monitoring and timely clinical intervention, making diagnostic processes more accurate and accessible. National programs, including the NIH’s Human Placenta Project, are advancing machine learning applications to non-invasively diagnose preeclampsia, highlighting AI as a catalyst for innovation in this market.
Preeclampsia Diagnostics Market Scope
| Report Coverage | Details |
| Market Size in 2025 | USD 1.29 Billion |
| Market Size in 2026 | USD 1.33 Billion |
| Market Size by 2034 | USD 1.70 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 3.10% |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2025 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Technology, Delivery System, Route of Administration, Target Disease, Target Tissue, End-User, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
What opportunities and trends are shaping the future of the market?
How can point-of-care devices improve maternal outcomes?
These devices facilitate rapid, portable testing, allowing earlier detection in outpatient clinics and homes, essential for regions with limited laboratory infrastructure.
What regions offer the highest growth potential?
Asia Pacific is booming due to enhanced healthcare access, rapid urbanization, and maternal care services, with notable initiatives like Amazon India’s at-home testing.
Are data analytics platforms becoming influential?
They transform raw clinical data into actionable insights, allowing real-time maternal health monitoring and personalized risk stratification.
How important is integration with telemedicine?
The growth of home-based monitoring is accelerating due to telehealth technologies, reducing the need for frequent hospital visits, especially in rural areas.
What challenges does the market face?
A significant challenge remains in the limited clinical validation of novel biomarkers for early detection, such as sFlt-1/PlGF ratios and RNA-based signatures, across diverse ethnic populations. Regulatory complexities and inconsistent validation data slow widespread adoption. Additionally, cost pressures in developing and scaling advanced diagnostics, particularly in emerging markets, restrict broader accessibility.
What breakthroughs and company contributions impact the market?
-
Mayo Clinic Laboratories launched the PERA test in 2024, achieving 94% sensitivity in detecting preeclampsia, setting a new standard for diagnostic precision.
-
The Foundation for the National Institutes of Health (FNIH) initiated a public-private partnership to develop high-risk pregnancy identification tools.
-
Major players leading technological innovation include Thermo Fisher Scientific, F. Hoffmann La Roche Ltd., PerkinElmer Inc., Siemens Healthineers, Abbott Laboratories, and Bayer AG.
-
Emerging companies like Diabetomics, Inc. and Sera Prognostics focus on specialized assays and regional deployment strategies.
How does the regional landscape influence market dynamics?
North America dominates, largely due to its sophisticated healthcare ecosystem, strong research funding, and regulatory support for rapid diagnostic development. The U.S. market alone is forecasted to grow from USD 427.12 million in 2025 to USD 573.11 million by 2034. Conversely, Asia Pacific’s rapid growth is powered by healthcare infrastructure expansion and increasing maternal diagnostic awareness, with India and China being key contributors.
Key Players Operating in the Preeclampsia Diagnostics Market and Their Offering
| Tier | Companies in Tier | Approx % Cumulative Share | Commentary |
| Tier I – Market Leaders (~45 55%) | Thermo Fisher Scientific; F. Hoffmann La Roche Ltd.; PerkinElmer Inc. | ~ 50% | These firms dominate via strong biomarker assay platforms (especially sFlt 1/PlGF), large global lab networks, regulatory approvals, and broad product portfolios. |
| Tier II – Established Players (~20 30%) | Siemens Healthineers; Abbott Laboratories; Bayer AG; QuidelOrtho Corporation | ~ 25% | These contribute significantly via diagnostics instruments, rapid/point of care platforms, complementary assays, and expanding reach into emerging markets. |
| Tier III – Emerging / Niche Players (~15 20%) | DRG Instruments GmbH; Diabetomics, Inc.; Metabolomic Diagnostics Ltd.; Sera Prognostics | ~ 15% | These smaller or more specialized companies often focus on risk score tools, early detection/prognostic assays, or regional deployment. |
Segments Covered in the Report
By Test Type
- Blood Pressure Monitoring
- Biochemical Marker Assays
- Placental Growth Factor (PlGF)
- sFlt-1 / sEng Ratio
- Others
- Urine Protein Tests
- Imaging-Based Diagnostics (Ultrasound / Doppler)
- Point-of-Care Testing
- Others
By Technology Type
- Immunoassays
- ELISA / Chemiluminescence-based
- Molecular / PCR-based Assays
- Biosensors / Point-of-Care Devices
- Ultrasound / Imaging-based
- Others
By Test Setting
- Laboratory-based Testing
- Point-of-Care / Bedside Testing
- Home-based / Remote Monitoring
By Product & Services
- Diagnostic Kits & Reagents
- Testing Devices & Instruments
- Laboratory Services
- Data Analytics & Monitoring Platforms
- Others
By End User (no sub-segments)
- Hospitals & Maternity Clinics
- Diagnostic Laboratories
- Obstetrics & Gynecology Clinics
- Research & Academic Institutes
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Read Also: RNA Interference Drug Delivery Market
You can place an order or ask any questions. Please feel free to contact us at sales@precedenceresearch.com | +1 804 441 9344