Why Stabilize Chromium?
-
Chromium (Cr³⁺ and Cr⁶⁺) is a heavy metal widely found in industrial waste from leather tanning, electroplating, steel production, and chemical manufacturing.
-
While Cr³⁺ is less toxic than Cr⁶⁺, under certain environmental conditions it can oxidize into the highly toxic and carcinogenic hexavalent form (Cr⁶⁺).
-
To prevent this, scientists focus on immobilizing Cr³⁺ in forms that are chemically stable, water-insoluble, and resistant to environmental changes.
This is where phosphate cement comes in.
The Innovation: Iron-Slag-Derived Phosphate Cement
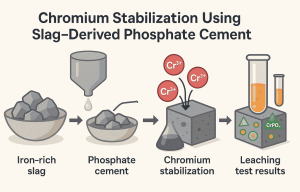
The study introduced a phosphate cement made from industrial byproduct—iron-rich slag (a waste material from steel production). Instead of disposing of slag, researchers used it to create a cement binder that can lock chromium inside its structure.
Process Overview
-
Raw Material: Iron-rich slag is combined with phosphoric acid to form phosphate cement.
-
Chromium Addition: Chromium in the form of Cr³⁺ is introduced during cement preparation.
-
Stabilization Mechanism:
-
Cr³⁺ reacts with phosphate ions, forming chromium phosphate (CrPO₄) crystals.
-
Some Cr³⁺ enters into co-precipitates with iron, creating mixed chromium–iron phosphate phases.
-
These phases are physically encapsulated in the dense cement matrix, making them resistant to leaching.
-
Download the Sample Copy of the Report: https://www.precedenceresearch.com/sample/6652
Key Findings from the Study
-
Formation of Stable Phases
-
X-ray diffraction (XRD) and electron microscopy showed the presence of crystalline CrPO₄ and mixed Fe–Cr–PO₄ compounds.
-
These structures are stable under environmental pH conditions.
-
-
Superior Immobilization Efficiency
-
Leaching tests (simulating acid rain and groundwater exposure) confirmed that >95% of chromium remained immobilized.
-
Chromium concentrations in leachates were below regulatory limits.
-
-
Synergy with Iron
-
The iron in slag plays a dual role:
-
It provides structural strength to the cement.
-
It participates in co-precipitation, strengthening chromium’s fixation.
-
-
-
Cost-Effectiveness & Sustainability
-
Since slag is an industrial waste, using it for cement is both economical and eco-friendly.
-
The process converts two waste streams (slag and chromium-contaminated residues) into a stable, safe material.
-
Why This Matters
-
Environmental Remediation: This approach can be applied in polluted soil, sludge, and wastewater treatment, especially in areas near steel plants and tanneries.
-
Circular Economy: Turns industrial byproducts into functional materials instead of landfill waste.
-
Long-Term Safety: Locks chromium in mineral-like structures, reducing the risk of future contamination.
Real-World Applications
-
Stabilization of chromium-rich sludge from electroplating factories.
-
Safe disposal of tannery waste containing Cr³⁺.
-
Cement-based solidification for contaminated construction materials.
-
Potential expansion to stabilize other heavy metals like lead, cadmium, and arsenic.
This study demonstrates a breakthrough in green remediation technologies. By leveraging slag-derived phosphate cement, chromium can be converted into stable chromium phosphate and iron–chromium phosphate co-precipitates, effectively immobilizing the metal and preventing its re-entry into the environment. The approach is low-cost, sustainable, and scalable, making it highly relevant for industries and governments tackling heavy metal pollution.
You can place an order or ask any questions, please feel free to contact us at sales@precedenceresearch.com |+1 804 441 9344