The cell and gene therapy quality control and analytics market stands at the forefront of the next healthcare revolution. Growing at a remarkable compound annual growth rate (CAGR), with Asia Pacific leading at approximately 33.5%, this sector is driven by surging cases of genetic diseases, relentless innovation in gene editing, and real-time, AI-enabled analytics.
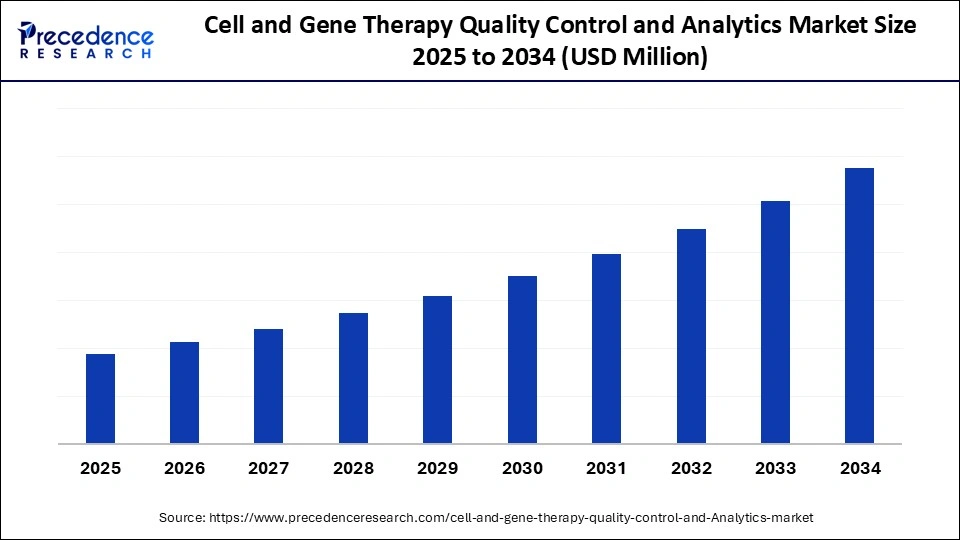
North America commands about 43% of the global market, while advancements in rapid testing and automation continue to reshape industry expectations.
Cell and Gene Therapy Quality Control and Analytics Market Key Insights
-
The market’s dominating region is North America, capturing about 43% of the global share in 2024, driven by strong infrastructure and regulatory frameworks.
-
Asia Pacific is the fastest-growing region, expected to register a 33.5% CAGR through 2034 due to significant research and government investments.
-
The sterility testing segment led all testing types in 2024, claiming roughly 24% market share as quality assurance becomes paramount.
-
PCR-based analytical methods accounted for around 28.6% market share, thanks to their rapid and sensitive profiling capabilities.
-
Pharmaceutical & biotechnology companies represented the largest end-user group (54.2% market share in 2024).
-
Tier I leaders include Thermo Fisher Scientific (AI-driven analytics, rapid diagnostics), Lonza, Merck KGaA, Bio-Rad Laboratories, and QIAGEN, with consistent advancements in automation and predictive analytics.
Cell and Gene Therapy Quality Control and Analytics Market Revenue and Segment Tables
| Segment | 2024 Market Share | Key Growth Drivers |
|---|---|---|
| North America (Region) | 43% | Advanced infrastructure, regulatory support |
| Asia Pacific (Region) | N/A | High CAGR, public investment |
| Sterility Testing (Testing) | 24% | Demand for robust quality assurance |
| PCR (Analytical Method) | 28.6% | Sensitivity, rapid turnaround |
| Oncology (Application) | 37.4% | Cancer prevalence, new CAR-T therapies |
| Pharma/Biotech (End-User) | 54.2% | Increased clinical and commercial activities |
Artificial intelligence has become the backbone of transformational change in cell and gene therapy quality control. AI’s role extends from predictive analytics and automated real-time monitoring to enhanced data integration across complex manufacturing processes. AI-driven solutions accelerate sterility assessments and potency testing, minimizing human error and generating unprecedented efficiencies in compliance and release protocols. Advanced AI platforms now support adaptive quality control, improving the reliability and scalability of cell and gene therapy manufacturing.
Integration of machine learning and digitalization also empowers companies to identify patterns within massive datasets from next-generation sequencing, PCR, and flow cytometry—optimizing testing algorithms for novel therapies. The competitive advantage is increasingly defined by organizations’ abilities to harness these technologies for faster approvals and safer, more consistent therapeutic products.
Cell and Gene Therapy Quality Control and Analytics Market Growth Factors
-
The increasing prevalence of chronic and genetic diseases intensifies demand for personalized therapeutics and rigorous quality control.
-
Rapid expansion of gene editing methods, including CRISPR-Cas9 and CAR-T platforms, necessitates state-of-the-art analytics.
-
Advancements in real-time control, single-cell analysis, and organ-on-a-chip technologies are unlocking new standards in testing accuracy and speed.
-
Market leaders are investing heavily in building robust quality control infrastructure and specialized services.
What New Market Opportunities and Trends Are Emerging?
How are partnerships accelerating validation and adoption of new QC technologies?
strategic collaboration between biopharmaceutical firms, CDMOs, tech innovators, and academia are paramount. These alliances enable rapid, regulatory-compliant validation of AI-driven analytics, rapid sterility testing, and real-time monitoring systems. Such partnerships foster scalable and efficient QC solutions and reduce both regulatory and financial barriers for new therapy launches.
What breakthroughs are expected to reshape the competitive landscape?
The surge in advanced sterility and potency assays, real-time digital platforms, and mass spectrometry-driven analytics is transforming speed and accuracy. Innovations from Thermo Fisher Scientific—especially in automation and AI are setting higher standards in consistency and cost-efficiency. Emerging tech like microfluidic systems and organ-on-a-chip solutions are on track to disrupt traditional QC models.
Cell and Gene Therapy Quality Control and Analytics Market Regional and Segment Analysis
North America leads with about 43% of market share in 2024, benefiting from an unmatched healthcare infrastructure, strong regulatory adherence, and seamless integration of digital and AI technologies. The U.S. further supports market growth with robust R&D investment, rising prevalence of chronic diseases, and a prolific clinical pipeline.
Asia Pacific is the fastest-growing region, spurred by state-sponsored R&D, a large and aging population, and rising incidence of chronic and rare diseases, particularly in China. Government spending and vibrant clinical trial activity continuously drive the adoption of QC solutions.
Europe leverages strong EMA support and advanced gene editing initiatives in Germany and the UK, focusing on regulatory-compliant innovation and extensive outsourcing for QC services.
By Testing Type: Sterility testing holds the dominant share at 24%, while potency testing is expanding rapidly (21.5% CAGR).
By Analytical Method: PCR leads with 28.6% market share; mass spectrometry sees ~23.8% CAGR boost.
By Application Area: Oncology is the top application, holding a 37.4% share; genetic disorders are a prime growth focus.
By End-User: Pharma and biotech companies claim the majority with 54.2%; CDMOs are rapidly rising, empowered by regulatory complexity and demand for scalability.
Key Companies and Latest Breakthroughs
Market Leaders (Tier I)
-
Thermo Fisher Scientific – Next-generation sequencing, PCR/qPCR, AI-based analytics, automated QC
-
Lonza Group – Integrated quality control and manufacturing
-
Merck KGaA – Analytical tech and QC consumables
-
Bio-Rad Laboratories – Precision instruments and QC reagents
-
QIAGEN – Molecular analytics solutions
Established Players (Tier II)
-
Charles River Laboratories, Bio-Techne, WuXi AppTec, Catalent, Eurofins Scientific
Emerging and Niche Innovators (Tier III)
-
IQVIA, F. Hoffmann-La Roche, Samsung Biologics, Sangamo Therapeutics, Galapagos NV
The latest wave of breakthroughs revolves around AI-powered analytics, real-time monitoring, rapid sterility/potency testing, and integration of digital and microfluidic technologies to expedite compliant, efficient therapy release.
Challenges and Cost Pressures
The industry faces mounting challenges in managing advanced product complexity, high setup costs, and fast-evolving regulatory requirements. Pressure to deliver scalable, error-minimized, and cost-effective solutions compels companies to pursue digital transformation and workflow automation aggressively. Ensuring consistent global compliance without raising costs is now central to both operational and strategic decision-making.
Case Study: Transforming Oncology QC with AI
A leading U.S. biotech company recently implemented an AI-driven analytics suite for real-time QC of its CAR-T therapy pipeline. The result: A 40% reduction in batch release times and improved compliance with FDA guidelines, achieved by seamlessly integrating predictive analytics and automated testing platforms at each stage of development. This win is rapidly influencing other oncology-focused QC models.
Read Also: Vivo Cell Reprogramming Market
You can place an order or ask any questions. Please feel free to contact us at sales@precedenceresearch.com |+1 804 441 9344